
SCROLL
Nitrogen: Friend or Foe?
Exposures in homes with intermittent peaks of NO2 have been associated with numerous concerns, including severe asthma in children and adults.
BUILDING HEALTH: A PHYSICIAN'S VIEW

There are many homes in which gas stoves cause short-term “peak” exposures that result in higher indoor NO2 levels than can be found outdoors near highways.
Photo by Mykola Makhlai on Unsplash
Nitrogen serves many purposes, some good and some not so good. Here is a look at this key element from both sides of the fence.
Friend: Our cells need nitrogen.
Nitrogen (N), one of the four most abundant elements in our atmosphere — along with hydrogen, carbon, and oxygen — is an essential element for all living organisms. A structural component of proteins involved in carrying our genetic code as well as maintaining our cells, enzymes, and hormones, we could not live without it.
Nitrogen gas (N2) makes up 78% of our inhaled air, yet this gas is so stable (inert) due to the triple bond that our tissues cannot utilize the respired form. Instead, we depend on plants and microbes to break inert N2 gas apart, a process known as nitrogen fixation, to create a more reactive state we access through eating plants or animals that have consumed nitrogen rich vegetation. This complexity of nitrogen molecule derivatives is due to multiple configurations, or oxidation states, allowed by the five electrons swarming around the core of protons and neutrons.
Foe: Human diseases from anthropogenic sources of excess nitrogen dioxide.
Examples of high-energy chemical reactions that fix nitrogen, or split the N2 bond, are associated with motor vehicles, domestic gas stoves, water heaters, and kerosene space heaters. The gas mixtures released in these high-energy reactions contain nitrogen dioxide (NO2), an acidic and highly corrosive gas that can cause serious harm to our health.
When water-soluble gases (which nitrogen dioxide is not) are inhaled, they rapidly dissolve into upper airway mucus and irritate the throat, alerting the person to quickly distance themselves from the exposure.
In contract, when low-solubility NO2 is inhaled, no immediate cough reaction from upper airway irritation is triggered, allowing the toxic gas to reach deep layers of the lungs before a danger is perceived. NO2 and reaction products subsequently either remain within the lung or reach the circulatory system. Once in the blood, NO2 can bind to the oxygen carrying molecule hemoglobin to form methemoglobin (MetHb) — an ineffective oxygen carrier. The transformation to MetHb exacerbates hypoxia (low oxygen states) in people who have underlying respiratory or cardiac disease.
Homes with combustion appliances may have low levels of indoor air pollutants, especially when exposures are averaged over months or years, but there are many homes in which gas stoves cause short-term “peak” exposures that result in higher indoor NO2 levels than can be found outdoors near highways.
Exposures in homes with intermittent peaks of NO2 have been associated with more severe asthma in children and adults, exacerbation of chronic obstructive pulmonary disease, increased severity of respiratory infection, lower birth weight in newborns, and increased risk of premature death.
Case study: Three hours of IAQ monitoring in a “high-end” New England home, built in 2019
Sensors installed, continuous monitoring of temp., RH, CO, NO2, SO2, tVOCs, PM 2.5, CO2, O3
Findings:
5 p.m. — Kitchen tVOC and NO2 levels elevated during cooking, family room fireplace not in use, humidifier off;
6 p.m. — family room fireplace in use, humidity low, particles and carbon monoxide elevated, kitchen pollutants drawn into family room (probably due to negative pressure from fireplace flue); and
7 p.m. — humidifier on, particles and tVOCs reduced.
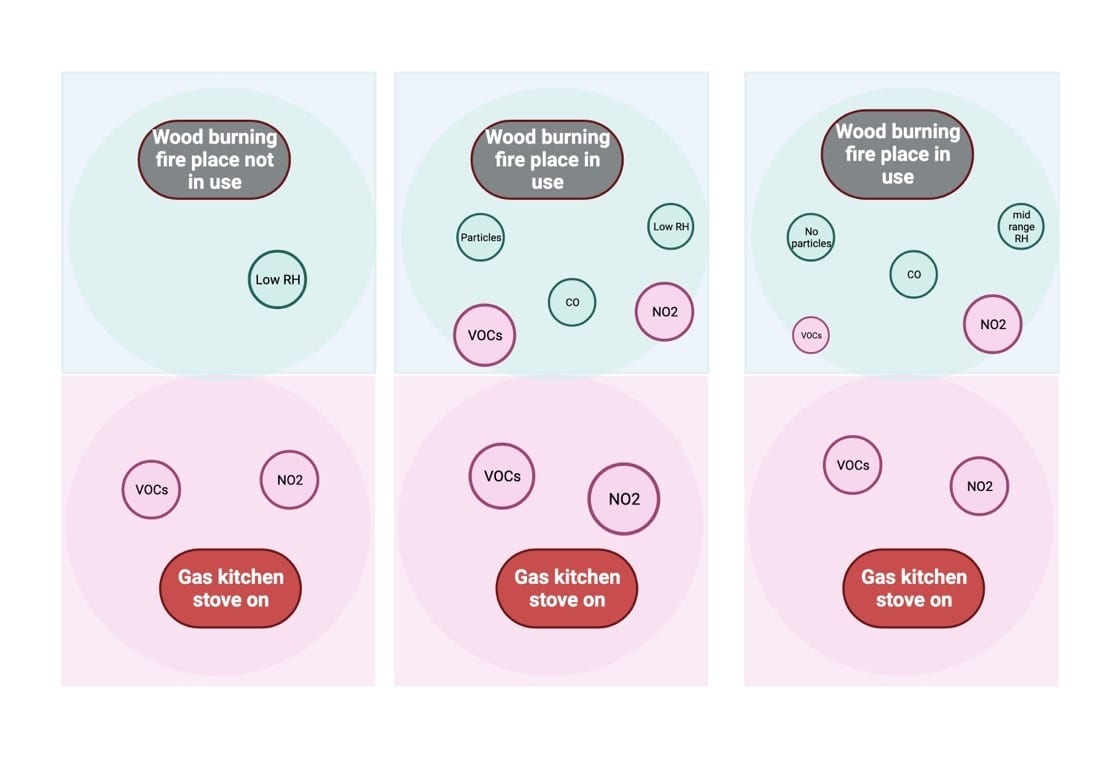
FIGURE 1. A bubble diagram showcasing the floor plans of adjacent kitchen and family rooms pertinent to the case study.
Image courtesy of Building4Health
Recommendations for IAQ management in this case study home:
- Switch to electric stove (if desired);
- Vent gas stove more effectively;
- Double venting using nearby bathroom fan if using fireplace while cooking;
- Use family room humidifier to maintain RH 40%-60%;
- Keep baby or asthmatic people out of family room if cooking and using fireplace; and
- Increase ventilation in family room to prevent negative pressure from fireplace flue.
Public steps in place to address potential health impact of gas stoves:
By March 1, 2023, Consumer Product Safety Commission staff intends to submit a request for information (RFI) to seek public input on hazards associated with gas stoves and proposed solutions to those hazards.
- Requirements for range hoods that meet mandatory performance standards, assessing their efficiency of removing the pollutants;
- Where feasible, issue performance standards for gas stoves that address steady-state-off leakage, including requiring automatic shut-off valves;
- Where feasible, issue performance standards for gas stoves that address the health impacts of hazardous emissions;
- Require labels on gas stoves that educate consumers about their exposure risks; and
- Launch a public education campaign on the health risks of cooking with a gas stove and offer steps consumers can take to minimize their risk.
