
SCROLL
FEATURE
HVAC Design Considerations for Pharmaceutical Preparations
Air cleanliness, space pressurization, required air changes, and equipment placement are just a few of the many important factors that must be considered.
SCROLL
Pharmaceutical suites are subject to strict requirements regarding the air supplied to and exhausted from their preparation, storage, and distribution areas. Designing for an appropriate level of pressurization and filtration for a given pharmaceutical preparation space is imperative to ensuring the space properly protects the personnel, products, and environment from contaminants.
The strictest environmental requirements in a pharmaceutical suite are reserved for compounding rooms. Per the U.S. Pharmacopeia (USP), compounding includes the preparation, mixing, assembly, alteration, packaging, and labeling of nonhazardous drugs (NHDs) and hazardous drugs (HDs). Anterooms abut compounding rooms and are used to prevent contaminants from spreading to unwanted areas or rooms. Figure 1 depicts an example layout of compounding rooms and anterooms in a pharmaceutical suite.
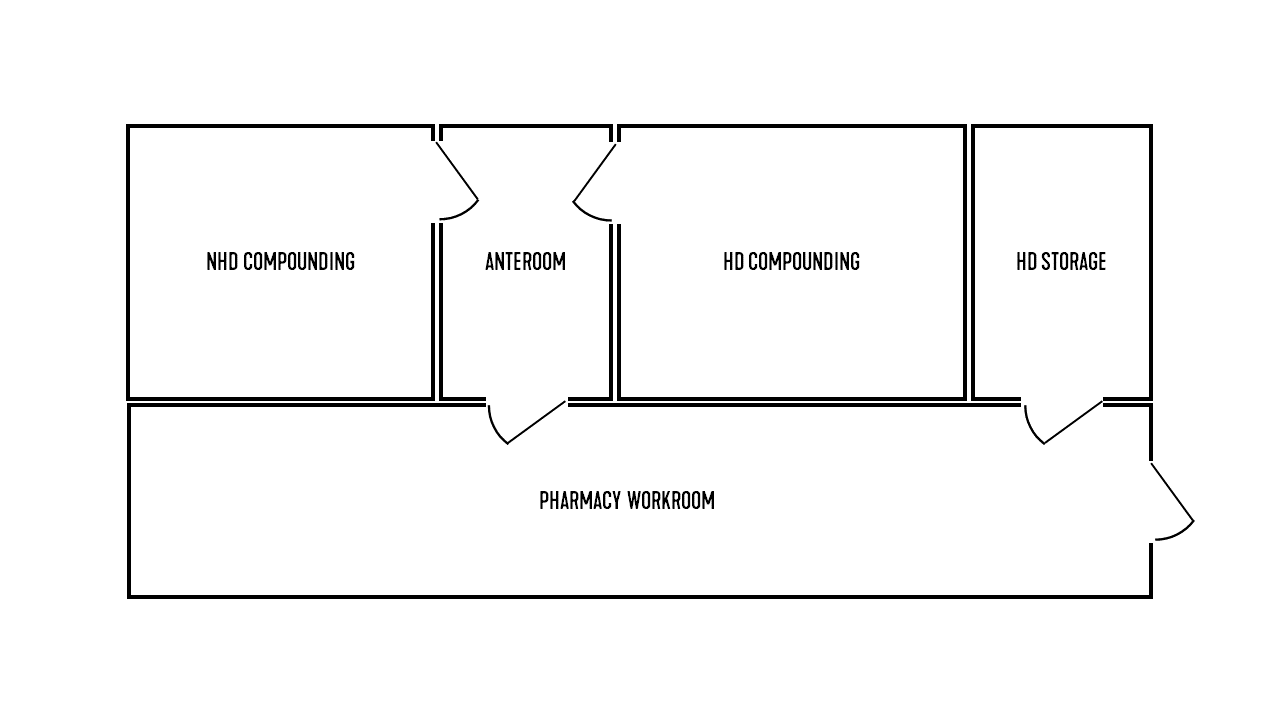
FIGURE 1: An example layout of pharmaceutical preparation areas.
Images courtesy of SmithGroup
The USP developed three sets of standards that dictate proper preparation conditions for compounded drugs. These three standards cover four permutations of hazard level and sterility, compounded sterile preparations (CSPs), and compounded nonsterile preparations (CNSPs) of both NHDs and HDs. The hazard level and sterility requirements of a compounded drug directly affect the air cleanliness level, space pressurization, and air changes per hour (ACH) required in the compounding room. A breakdown of which standards dictate which preparations is shown in Figure 2.
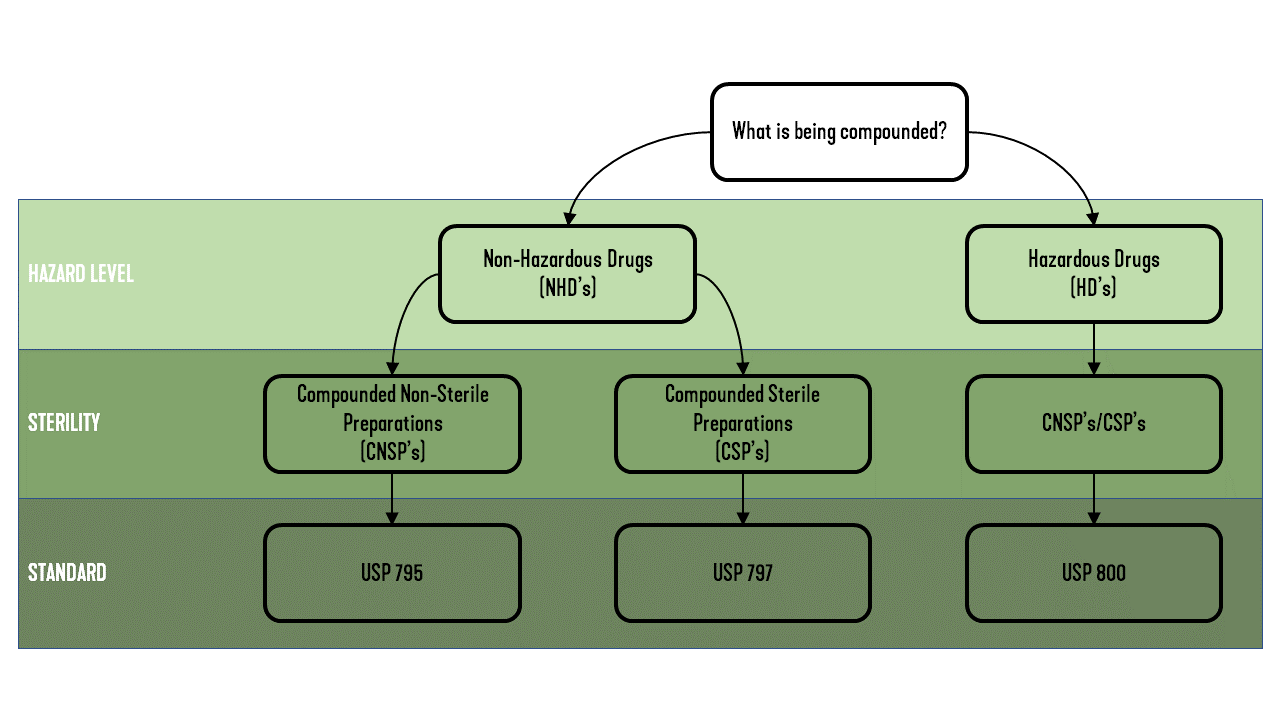
FIGURE 2: The governing standard based on drug preparation.
USP 795, “Pharmaceutical Compounding – Nonsterile Preparations,” outlines the proper preparation conditions for CNSPs. USP 795 is the least restrictive and states only that the HVAC system needs to be “controlled to avoid decomposition and contamination of chemicals.” Per USP 795, “temperature and humidity should be maintained as required for certain components and compounded dosage forms.” USP 795 also directs that the same compounding room cannot be used for CNSPs and CSPs.
USP 797, “Pharmaceutical Compounding – Sterile Preparations,” outlines the proper preparation conditions for CSPs. The air cleanliness classification system in Table 1 was set forth by the International Organization for Standardization (ISO) and by former U.S. Federal Standard 209E. USP 797 cites an ISO-classified air environment as one of the differences between the standards for sterile and nonsterile preparations.
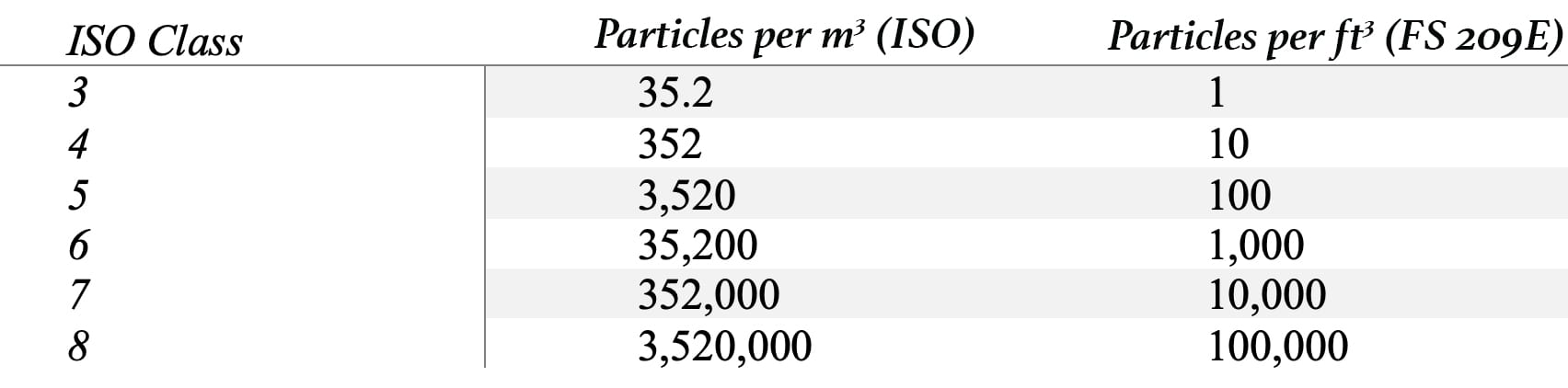
TABLE 1: Air cleanliness classifications per ISO and U.S. FS 209E.
CSPs are compounded inside a primary engineering control (PEC). USP 797 defines a PEC as a “device or room that provides an ISO Class 5 environment for the exposure of critical sites when compounding CSPs.” A few examples of PECs are laminar airflow workbenches (LAFW’s), biological safety cabinets (BSCs), and compounding aseptic containment isolators (CACIs). The compounding room that hosts the PEC is held to an ISO Class 7 environment and acts as a buffer area for the PEC. For NHDs, the PEC can recirculate air to the buffer area. The air exiting the PEC is considered suitable for the sterile environment because it passes through a bank of high-efficiency particulate air (HEPA) filters. Per USP 797, the air cleanliness requirement is at least ISO Class 8 for an anteroom serving a sterile NHD compounding room. If the buffer area and anteroom are physically separated — the USP-recommended configuration — the pressure differential must be held to a minimum of 0.02-0.05 inches water column (in. wc) to minimize the spread of contaminants. For sterile NHDs, USP 797 maintains that ISO Class 7 compounding rooms and their corresponding anterooms shall attain 30 ACH. When running, the PEC provides ACHs and recirculates filtered air into the space. Under this condition, the supply air can be reduced to a minimum of 15 ACH so long as the total ACH still reaches 30.
USP 800, “Hazardous Drugs – Handling in Healthcare Settings,” sets the environmental standards for the preparation of HDs. HDs must be prepared in an ISO Class 5 environment. The containment primary engineering control (C-PEC) used for HD preparation is commonly either a BSC or CACI and must be located within a containment secondary engineering control (C-SEC). The C-SEC, typically the compounding room itself, must be externally exhausted and negatively pressurized between 0.01-0.03 in. wc relative to all the surrounding spaces.
For nonsterile HDs, USP 800 indicates the C-PEC must be either externally vented or exhausted through redundant HEPA filters in series. The USP indicates a preference for externally vented C-PECs. The C-SEC must have a minimum exhaust of 12 ACH but does not need to meet an air cleanliness requirement. For sterile HDs, the C-PEC must be externally vented. The C-SEC must have a minimum supply of 30 ACH and needs to achieve an ISO Class 7 environment. For both sterile and nonsterile HD preparations, the C-SEC must be externally vented, negatively pressurized, and have a pressure differential of 0.01-0.03 in. wc relative to all adjacent areas that meet an ISO classification level. For sterile applications, the adjoining anteroom must also meet ISO Class 7 standards and be supplied at 30 ACH. If that anteroom is accessible from a room with no air cleanliness specification, a positive differential pressure of at least 0.02 in. wc is required with respect to the unclassified space.
Certain HDs, called antineoplastics, whether refrigerated or not, require a negatively pressurized storage room with a minimum exhaust of 12 ACH. Antineoplastic storage rooms do not require any other environmental controls. HDs that are not considered antineoplastics do not have any storage requirements that will affect the design of the HVAC system.
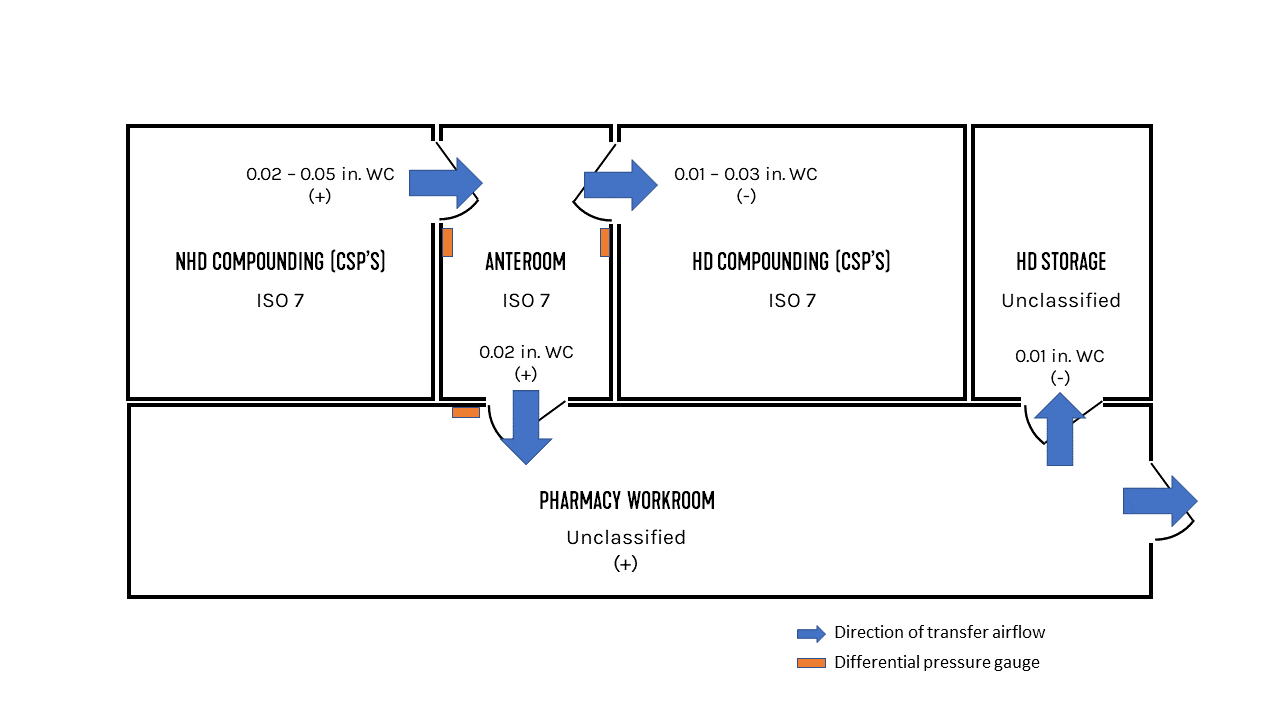
FIGURE 3: Pharmaceutical suite pressurization and airflow directions.
With an understanding of the requirements that apply to different preparations, the conditions of the example space from Figure 1 start to become clear. For this example, both the NHD and HD compounding rooms will perform only sterile preparations, and the HD storage room is storing antineoplastics.
The NHD compounding room needs to meet ISO Class 7 standards and must be positively pressurized between 0.02-0.05 in. wc. The positive pressurization helps to prevent air from entering the space. The HD compounding room also needs to meet ISO Class 7 standards and must be negatively pressurized between 0.01-0.03 in. wc. The HD storage room is unclassified with respect to air cleanliness, and because antineoplastics are present, a negative pressurization is required. A pressure differential of about 0.01 in. wc is recommended by these authors. The anteroom that serves both the NHD and HD compounding spaces must meet ISO Class 7 standards for air cleanliness, since it’s bordering a compounding room for sterile HDs. If it was bordering a compounding room for nonsterile HDs or for NHDs, an ISO Class 8 environment would be acceptable. Since the anteroom is adjoining a sterile HD compounding room and an unclassified room, it must be positively pressured to a differential of at least 0.02 in. wc with respect to the unclassified space. Adherence to all these guidelines results in a transfer airflow resembling the blue arrows in Figure 3.
For the example pharmaceutical suite, the required ACH are summarized in Figure 4. Pharmacy support spaces require a total of 4 ACH, as set by ASHRAE Standard 170. The pharmacy workroom is not required to meet an air cleanliness standard. ASHRAE Standard 170 does require that the workroom is positively pressured with respect to adjoining spaces, but it does not provide a minimum required pressure differential.
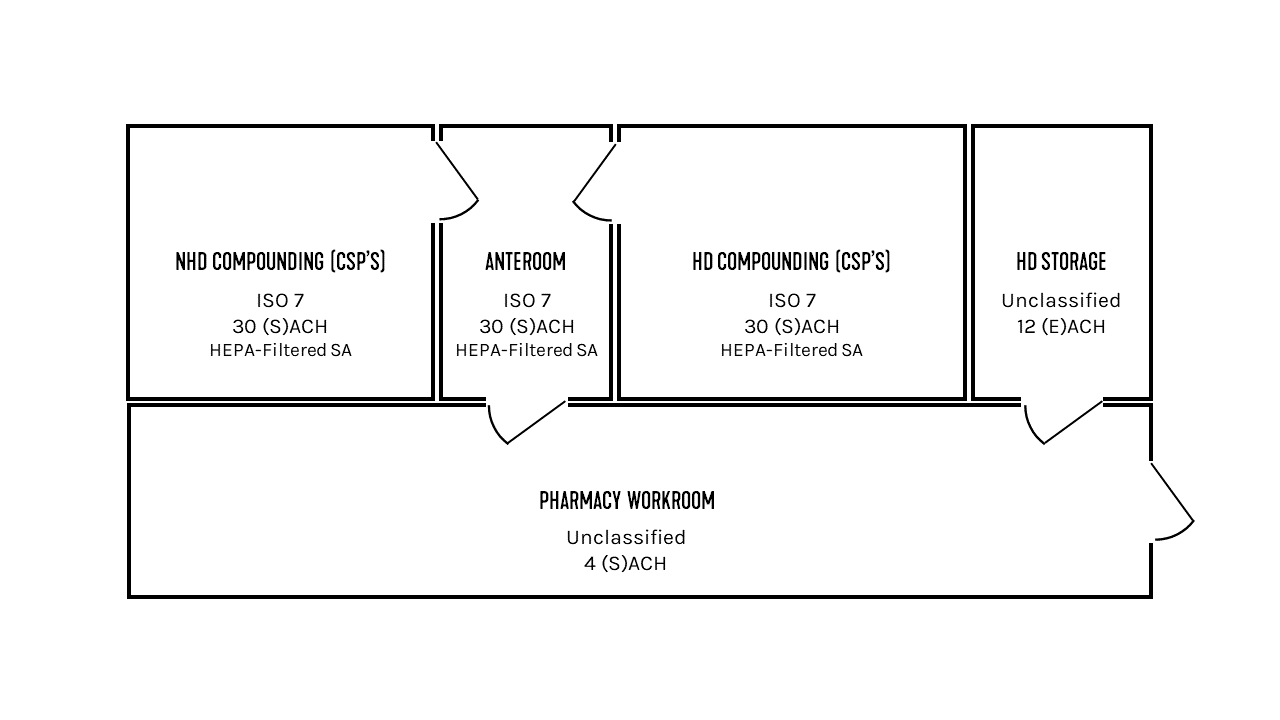
FIGURE 4: A summary of the required air changes.
Now that the space requirements have been identified, the system requirements can be considered. In order to effectively monitor the space pressurization requirements, USP 797 requires that “a pressure gauge or velocity meter shall be installed to monitor the pressure differential or airflow between the buffer area and ante-area and the ante-area and the general environment outside the compounding area.” Pressure gauges can be wall-mounted, and suggested mounting locations for the example pharmaceutical suite are highlighted in Figure 3. Psychrometric requirements in compounding rooms and anterooms are designed to achieve what is considered comfortable working conditions for personnel required to wear aseptic compounding garb. The target conditions are 68°F and between 35% and 60% relative humidity (RH).
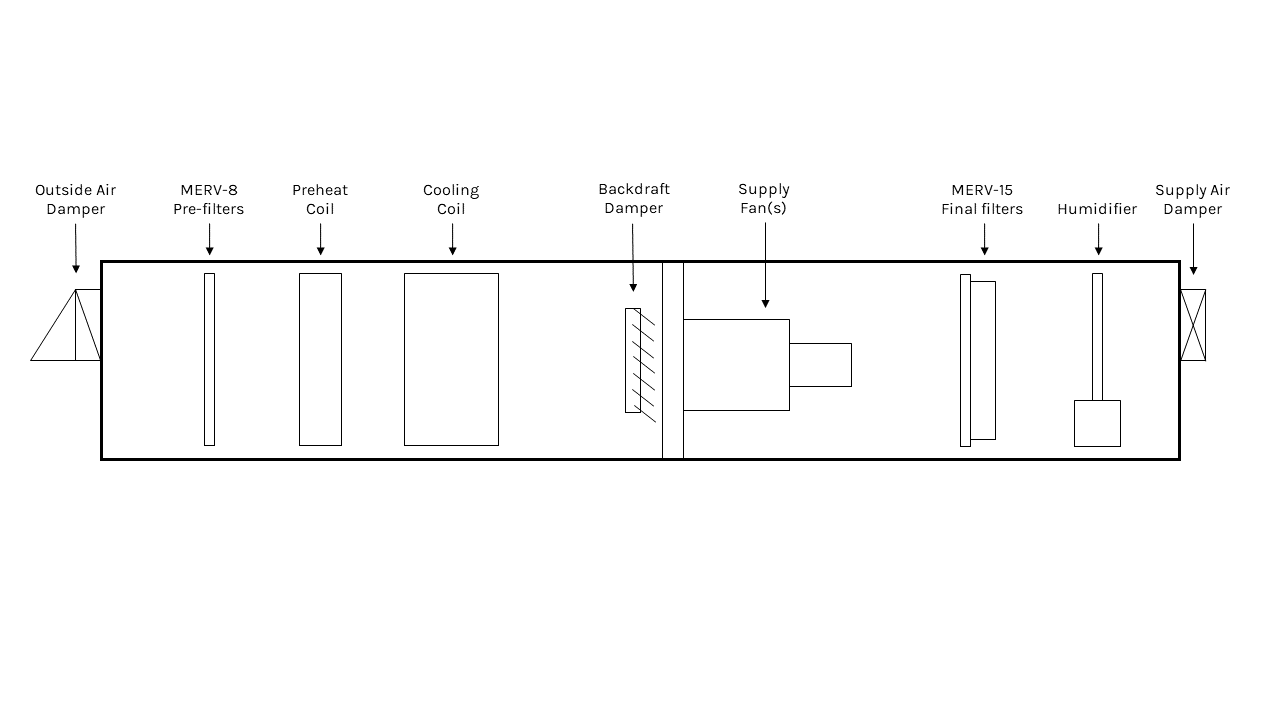
FIGURE 5: An example configuration of an AHU serving a pharmaceutical suite.
These authors recommends selecting a dedicated outdoor air system (DOAS) to feed compounding rooms and anterooms. Figure 5 depicts how a DOAS air-handling unit (AHU) that services the example pharmaceutical suite might look. The supply fan can be controlled by an airflow station to provide a constant airflow. A constant airflow set point is needed to ensure the required ACHs are maintained. A redundant supply fan is recommended by these authors, especially in applications where HDs are compounded. HEPA filters are utilized to achieve the level of air cleanliness for compounding rooms and anterooms. In the example pharmacy space, both compounding rooms and the anteroom need to be supplied with HEPA-filtered air. HEPA filters are very fine, and, because of this, the supply fan needs to work harder to push air through these filters. Thus, they are not used throughout the supply airstream and are often reserved for after the supply diffuser. HEPA filters have a very high minimum efficiency reporting value (MERV) of 17 or higher. Using a multi-filter system within the AHU, for example a pre-filter of MERV 8 and final filter of MERV 15, is more economical than using HEPA filters. This configuration reduces the load on the supply fan and mitigates wear on the more expensive HEPA filters located downstream. For humidification solutions, a steam humidifier can be installed after the final filters. This placement minimizes the risk of condensation on the filter, which could create mold issues. RH sensors located in the compounding rooms and anterooms monitor the RH and inform the steam control valves to modulate in order to maintain the minimum required 30% RH.
At the other end of the supply stream, variable-air volume (VAV) boxes can serve as terminal units in nonsterile compounding rooms and their associated anterooms as well as in general pharmacy work areas. Air valves are alternative terminal unit solutions that better suit sterile compounding rooms and their associated anterooms due to their faster response times. Air valves can rapidly respond to room conditions, such as changes in exhaust, resulting from PEC or C-PEC operation. Slower response times to significant changes in exhaust air, like those seen from a typical VAV box, could briefly compromise the space pressurization that is keeping harmful contaminants in the compounding room. USP 797 states that “supply air shall be introduced at the ceiling” and “air returns should be mounted low on the wall” for any room that is ISO Class 7 or cleaner. This is to encourage a downward airflow that will push contaminants away from preparation spaces and toward the floor.
While air from NHD compounding rooms and pharmacy support spaces is allowed to be returned, if the AHU is designed to handle return air, it cannot be returned from HD compounding rooms. Per USP 800, Tables 2 and 3, air from HD compounding rooms must be externally exhausted directly to the outside. Air externally exhausted from HD compounding rooms must pass through a bank of HEPA filters to protect the local environment from potentially hazardous chemicals. The exhaust must be discharged at a minimum of 10 feet above roof level.
Satisfying all the requirements of a pharmaceutical suite can be challenging, and improper scrutiny can have grave effect on the safety of pharmacy personnel and the viability of the drugs they produce. Air cleanliness, space pressurization, required air changes, and equipment placement are just a few of the many important factors that must be considered when designing the HVAC system for a pharmaceutical suite.

Thomas Strawderman
Thomas Strawderman is a mechanical engineer at SmithGroup. As an engineer in training, he is working on gaining diverse HVAC design experience in preparation for his licensing exams. Contact him at thomas.strawderman@smithgroup.com.
Ionel Petrus, P.E., CEM, LEED AP BD+C
Ionel Petrus is a licensed professional engineer who has more than 13 years of mechanical design experience. In addition to being the mechanical discipline leader in SmithGroup’s Washington, D.C., office, he is also a LEED AP and a certified energy manager. His experience includes designing HVAC systems for commercial buildings, research laboratories, health care facilities, and museums. He can be contacted at ionel.petrus@smithgroup.com.

[22gallery]/[Vetta] via Getty Images